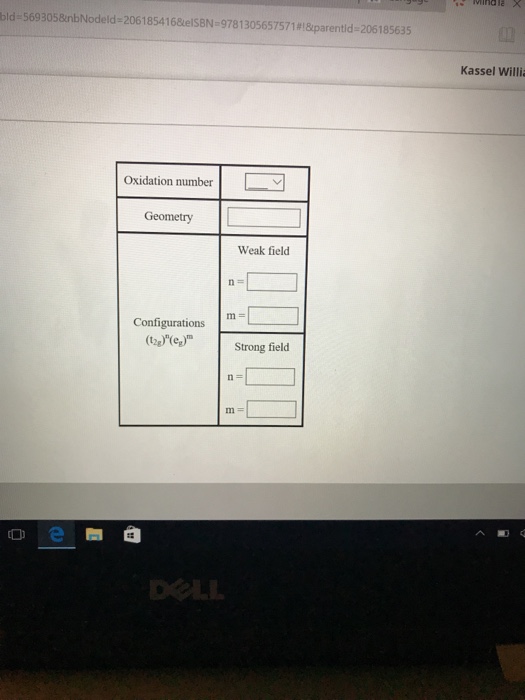
co[h2o]6cl2
The compound [Co(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of cobalt in this compound, the geometry of the coordination around the cobalt, and the possible configuration of the d electrons of the cobalt.

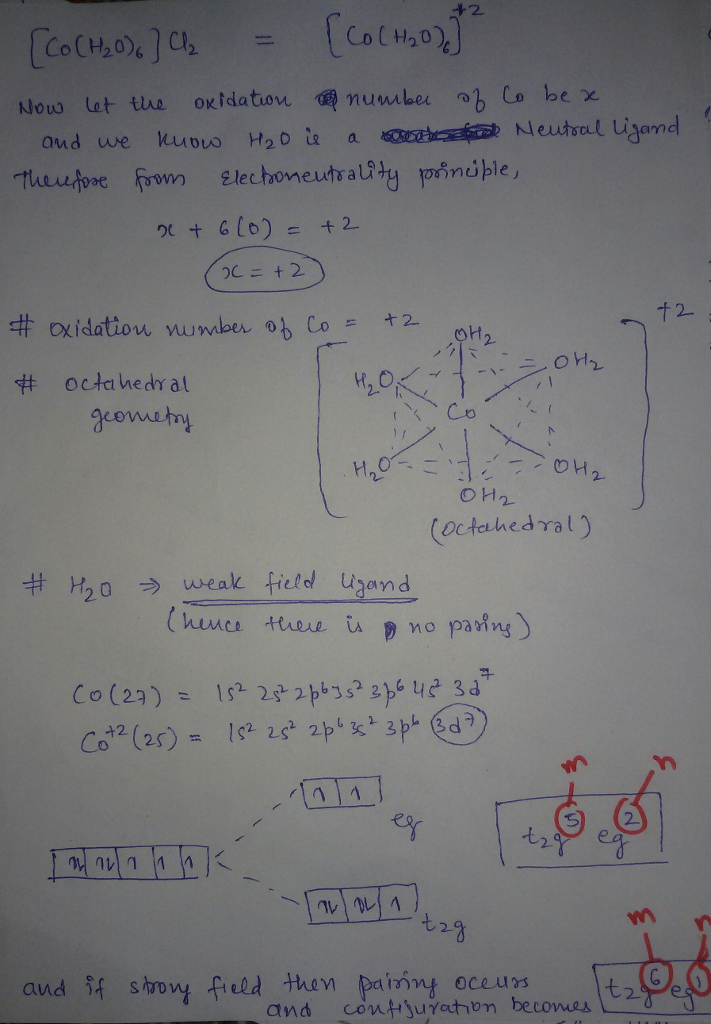 but H2O is weak fiels ligand hence configuration with weak field is TRUE
but H2O is weak fiels ligand hence configuration with weak field is TRUE
I had given you configuration as a strong ligand just because if you assumed it to be strong field. otherwise h20 is weak field ligand
co[h2o]6cl2
With us, you are either satisfied 100% or you get your money back-No monkey business


