in which of the following solutions will caf2 be least soluble?
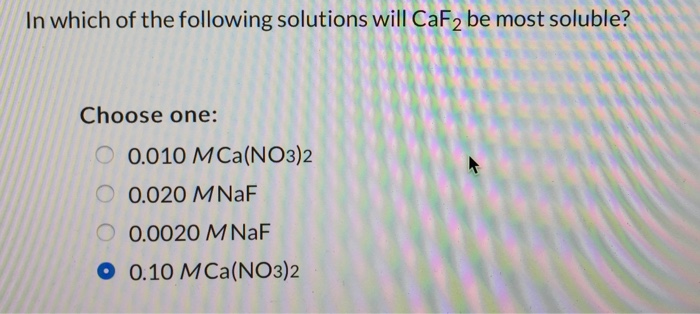
In which of the following solutions will CaF_2 be most soluble? Choose one: 0.010 M Ca(NO_3)_2 0.020 M NaF 0.0020 M NaF 0.10 M Ca(NO_3)_2

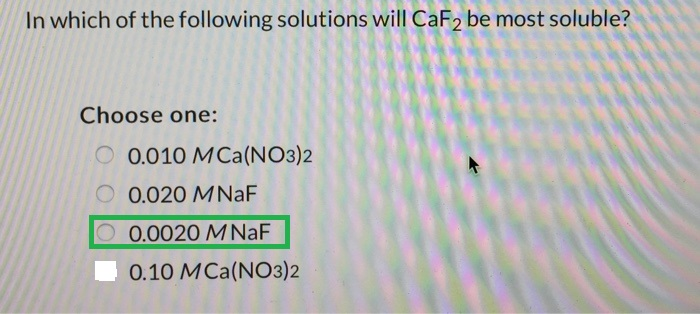
In which of the following solutions will CaF_2 be most soluble? Choose one: 0.010 M Ca(NO_3)_2 0.020 M NaF 0.0020 M NaF 0.10 M Ca(NO_3)_2
Here, the common ions present in the solution decrease the solubility of CaF2. The highest solubility will be observed in the solution with the lowest concentration
Answer: 0.0020 M NaF
With us, you are either satisfied 100% or you get your money back-No monkey business


