(b) O22-
(c) N22-
(d) F2+
(e) N2+
(f) O2+
(g) C22+
(h) Br22+
which of the following ions have electrons in pi antibonding orbitals
which of the following ions have electrons in pi antibonding orbitals
Which of the following molecular ions have electrons in π antibonding orbitals?
(a) O2–
(b) O22-
(c) N22-
(d) F2+
(e) N2+
(f) O2+
(g) C22+
(h) Br22+
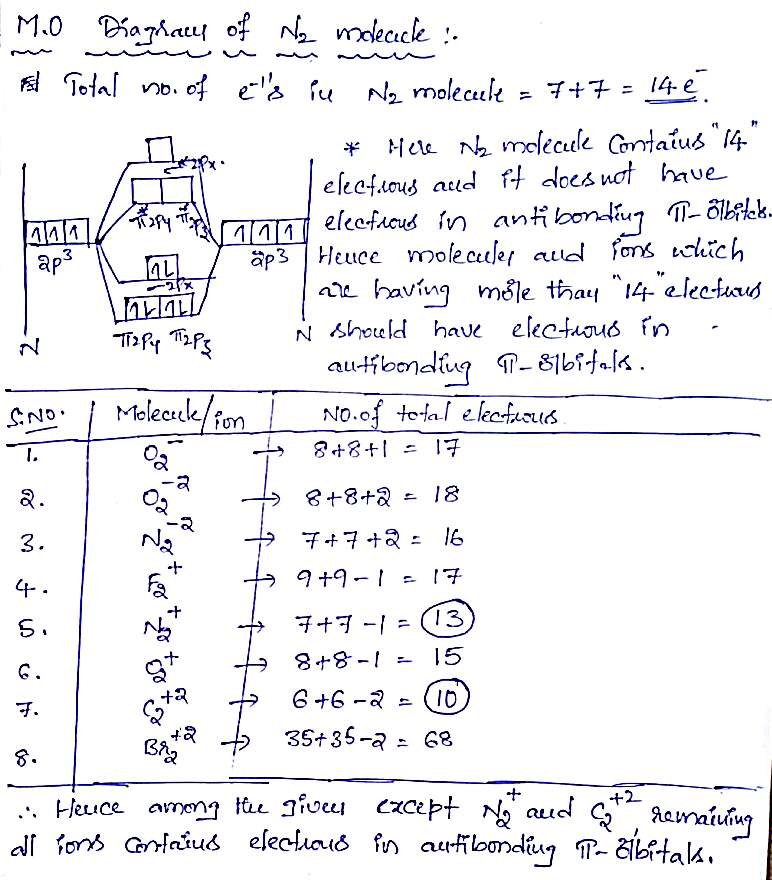
which of the following ions have electrons in π antibonding orbitals?
So much stress and so little time? We’ve got you covered. Get your paper proofread, edited or written from scratch within the tight deadline.
With us, you are either satisfied 100% or you get your money back-No monkey business


